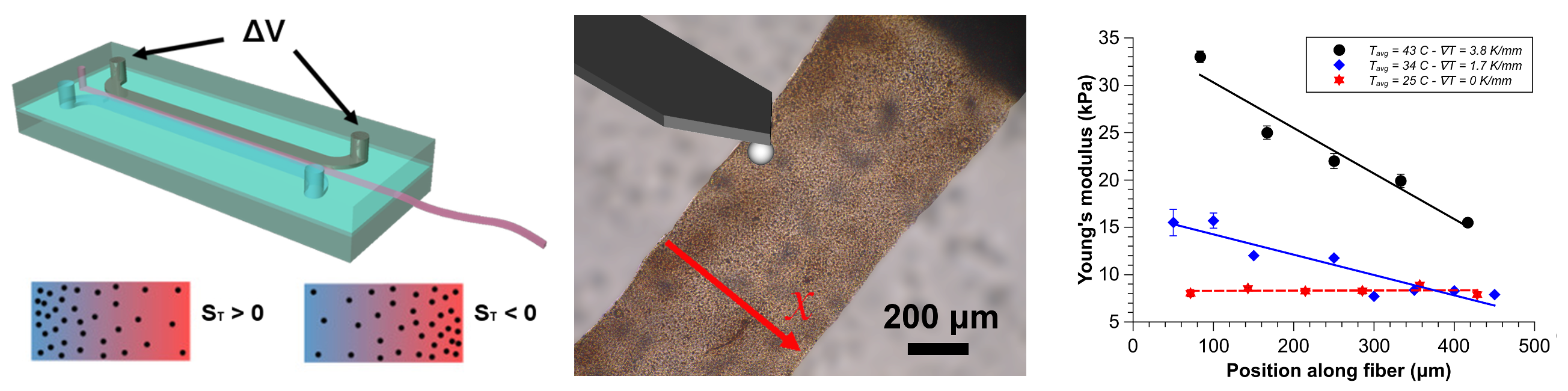
In the presence of a temperature gradient, any dispersed solute will experience a thermophoretic force, which will drive it to regions of high or low temperature, depending on its thermophoretic behavior.
Microfluidic devices, incorporating microchannels with cross-sectional dimensions of a few tens of microns, are ideal to exploit thermophoretic effects since temperature gradients can be established and controlled with high precision (e.g. through the use of micro Joule heaters). In particular by using biocompatible precursors (e.g. sodium alginate), it is possible to create substrates and fibers (high aspect ratio, wire-like objects) ideal for the study of cellular growth, motility or even diversification.
An aqueous solution of biocompatible hydrogel is made to flow in a microchannel with a transversal temperature gradient created by an embedded joule heater and a cold water flow. At the end of the microfluidic chip the formed hydrogel is extruded and collected.
The effective size of the extruded soft material is determined by the size of the microchannel and the flow rates used. In our preliminary experiments we were able to extrude strip of hydrogel of approximately 100 μm of thickness and up to 600 μm width.
The formed concentration gradient (induced by thermophoresis) is then “frozen” via crosslinking to create a solid (but soft) substrate that presents a gradient of mechanical properties (e.g. elasticity). Regions with different concentration of hydrogel precursor show different elastic behavior and thus a rigidity modulation.
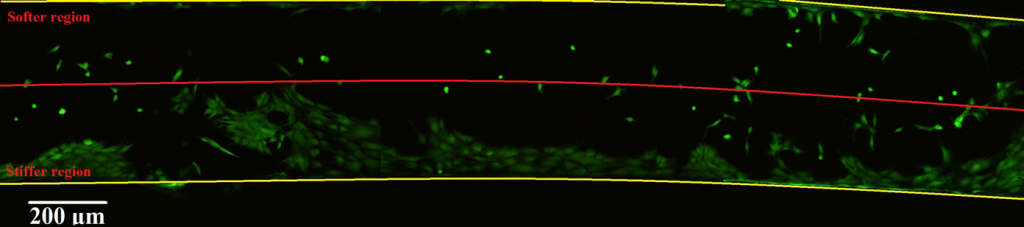
To finally confirm the capabilities of our functionalised biocompatible material, we grew osteoblasts on it and monitor their proliferation over time. As expected, the cells migrated towards the stiffer region following durotaxis. We further investigate mineralisation by XRF and found that a higher deposition of calcium and phosphorus is achieved at the stiffer side, again confirming the preference of osteoblasts to mineralise a substrate with a stiffness closer to the one of bones.