 Thermophoresis, akin to thermal diffusion in simple fluid mixtures, consists of particle drift induced by a temperature gradient.
Thermophoresis, akin to thermal diffusion in simple fluid mixtures, consists of particle drift induced by a temperature gradient.
When a macromolecular solution or a colloidal suspension is placed in a uniform-temperature gradient the dispersed particles migrate, focusing at either the cold or hot side. This effect, akin to thermal diffusion (the Soret effect) in simple fluid mixtures, is known as thermophoresis. In other words, the temperature gradient drives a flow of a solute that, in the absence of convection, leads to a steady-state concentration gradient.
A few notable contributions from our group
The riddle of particle size dependence
Several theoretical approaches to understand the relation between thermophoresis and size of the particles were made. The complexity of the problem is high to be easily and uniquely solved by use of standard procedures. That is why in the last years other theories were proposed that introduced a new characteristic length for thermophoresis, namely δ ≪ R, where R is the radius of the particle. In this thin layer the temperature dependence of the particle-solvent interfacial tension act generating particles motion. In consequence of this one can consider the thermophoretic velocity uT = DT ·∇T = D· ST ·∇T.
Our experiments confirmed the theory from Parola and Piazza stating that ST ∝ R.
First approach: Microemulsion droplets
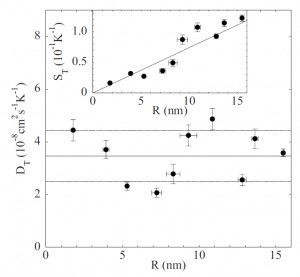 Notwithstanding its practical interest, the dependence of thermophoretic effects on particle size R is still theoretically and experimentally debated. By performing measurements of water-in-oil microemulsion droplets with tunable size, we show that the thermal diffusion coefficient, at least for a suspension of small particles in a nonpolar solvent, does not appreciably depend on R.
Notwithstanding its practical interest, the dependence of thermophoretic effects on particle size R is still theoretically and experimentally debated. By performing measurements of water-in-oil microemulsion droplets with tunable size, we show that the thermal diffusion coefficient, at least for a suspension of small particles in a nonpolar solvent, does not appreciably depend on R.
Publications:
D. Vigolo, G. Brambilla, and R. Piazza. Thermophoresis of microemulsion droplets: Size dependence of the Soret effect. Physical Review E, 75(4):40401, 2007.
Second approach: Polystyrene particles
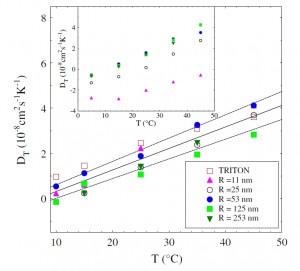 By measuring the full temperature dependence of this effect for polystyrene latex suspensions, we show that the thermophoretic mobility (or ‘‘thermal diffusion coefficient’’) DT is basically independent on particle size, in particular, when the interfacial properties of the colloidal particles are carefully standardized by adsorbing a surfactant layer on the particle surface. Even more, all investigated systems show values of DT which are very close to those measured for simple micellar solutions of the adsorbed surfactant. Our findings could be of relevance for downsizing microfluidics to the nanometric range.
By measuring the full temperature dependence of this effect for polystyrene latex suspensions, we show that the thermophoretic mobility (or ‘‘thermal diffusion coefficient’’) DT is basically independent on particle size, in particular, when the interfacial properties of the colloidal particles are carefully standardized by adsorbing a surfactant layer on the particle surface. Even more, all investigated systems show values of DT which are very close to those measured for simple micellar solutions of the adsorbed surfactant. Our findings could be of relevance for downsizing microfluidics to the nanometric range.
Publications:
M. Braibanti, D. Vigolo, and R. Piazza. Does thermophoretic mobility depend on particle size? Physical Review Letters, 100(10):108303, 2008.
Electrolytes effect on Thermophoresis
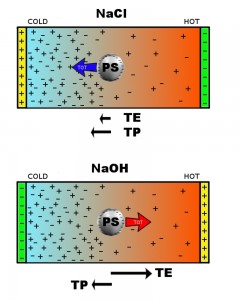 In electrolyte solutions, the differential migration of the ionic species induced by the presence of a thermal gradient leads to the buildup of a steady-state electric field. Similarly to what happens for the Seebeck effect in solids, the sample behaves therefore as a thermocell. Here, we provide clear evidence for the presence of thermoelectric fields in liquids by detecting and quantifying their strong effects on colloid thermophoresis. Specifically, by contrasting the effects of the addition of NaCl or NaOH on the Soret effect of micellar solutions of sodium dodecyl sulfate, we show that the presence of highly thermally responsive ions such as OH- may easily lead to the reversal of particle motion. Our experimental results can be quantitatively explained by a simple model that takes into account interparticle interactions and explicitly includes the micellar electrophoretic transport driven by such a thermally generated electric field. The chance of carefully controlling colloid thermophoresis by tuning the solvent electrolyte composition may prove to be very useful in microfluidic applications and field-flow fractionation methods.
In electrolyte solutions, the differential migration of the ionic species induced by the presence of a thermal gradient leads to the buildup of a steady-state electric field. Similarly to what happens for the Seebeck effect in solids, the sample behaves therefore as a thermocell. Here, we provide clear evidence for the presence of thermoelectric fields in liquids by detecting and quantifying their strong effects on colloid thermophoresis. Specifically, by contrasting the effects of the addition of NaCl or NaOH on the Soret effect of micellar solutions of sodium dodecyl sulfate, we show that the presence of highly thermally responsive ions such as OH- may easily lead to the reversal of particle motion. Our experimental results can be quantitatively explained by a simple model that takes into account interparticle interactions and explicitly includes the micellar electrophoretic transport driven by such a thermally generated electric field. The chance of carefully controlling colloid thermophoresis by tuning the solvent electrolyte composition may prove to be very useful in microfluidic applications and field-flow fractionation methods.
Publications:
D. Vigolo, S. Buzzaccaro, and R. Piazza. Thermophoresis and Thermoelectricity in Surfactant Solutions. Langmuir, 26(11):7792–7801, 2010.
Microfluidics Separator: an application of Thermophoresis
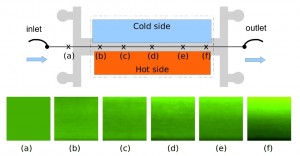 We show that thermophoresis, i.e., mass flow driven by thermal gradients, can be used to drive particle motion in microfluidic devices exploiting suitable temperature control strategies. Due to its high sensitivity to particle/solvent interfacial properties, this method presents several advantages in terms of selectivity compared to standard particle manipulation techniques. Moreover, we show that selective driving of particles to the cold or to the hot side can be achieved by adding specific electrolytes and exploiting the additional thermoelectric effect stemming from their differential thermal responsiveness.
We show that thermophoresis, i.e., mass flow driven by thermal gradients, can be used to drive particle motion in microfluidic devices exploiting suitable temperature control strategies. Due to its high sensitivity to particle/solvent interfacial properties, this method presents several advantages in terms of selectivity compared to standard particle manipulation techniques. Moreover, we show that selective driving of particles to the cold or to the hot side can be achieved by adding specific electrolytes and exploiting the additional thermoelectric effect stemming from their differential thermal responsiveness.
Publications:
D. Vigolo, R. Rusconi, H. A. Stone, and R. Piazza. Thermophoresis: microfluidics characterization and separation.Soft Matter, 6(15):3489–3493, 2010.
D. Vigolo, R. Rusconi, R. Piazza, and H. A. Stone. A portable device for temperature control along microchannels.Lab Chip, 10(6):795–798, 2010.
Giant thermophoresis of poly(N-isopropylacrylamide) (PNIPAM) microgel particles
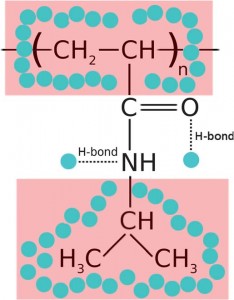 Thermophoresis is the rectification of Brownian motion induced by the presence of a thermal gradient ∇T, yielding a net drift of colloidal particles along or against the direction of ∇T. The effect is known to depend on the specific interactions between solute and solvent, and quantitative theoretical models are lacking except in a few simple experimental cases. Both the order of magnitude and the temperature dependence of the thermophoretic mobility DT are known to be very similar for a wide class of aqueous colloidal systems, ranging from latex colloids to polymers, surfactant micelles, proteins, and DNA. Here we show that thermoresponsive microgel particles made of poly(N-isopropylacrylamide) (PNIPAM) do not share, in the temperature range around the θ-point, these common features. Instead, DT displays an unusually strong temperature dependence, maintaining a linear growth across the collapse transition. This behaviour is not shared by linear PNIPAM chains, for which existing data show DT falling at the transition, with similar values between the expanded coil and collapsed globule states away from the transition point. A possible connection of the observed giant temperature dependence of DT to microgel hydration is suggested.
Thermophoresis is the rectification of Brownian motion induced by the presence of a thermal gradient ∇T, yielding a net drift of colloidal particles along or against the direction of ∇T. The effect is known to depend on the specific interactions between solute and solvent, and quantitative theoretical models are lacking except in a few simple experimental cases. Both the order of magnitude and the temperature dependence of the thermophoretic mobility DT are known to be very similar for a wide class of aqueous colloidal systems, ranging from latex colloids to polymers, surfactant micelles, proteins, and DNA. Here we show that thermoresponsive microgel particles made of poly(N-isopropylacrylamide) (PNIPAM) do not share, in the temperature range around the θ-point, these common features. Instead, DT displays an unusually strong temperature dependence, maintaining a linear growth across the collapse transition. This behaviour is not shared by linear PNIPAM chains, for which existing data show DT falling at the transition, with similar values between the expanded coil and collapsed globule states away from the transition point. A possible connection of the observed giant temperature dependence of DT to microgel hydration is suggested.
Publications:
S. Wongsuwarn, D. Vigolo, R. Cerbino, A. M. Howe, A. Vailati, R. Piazza, and P. Cicuta. Giant thermophoresis of poly(N-isopropylacrylamide) microgel particles. Soft Matter, 8(21):5857–5863, 2012.
Continuous Isotropic-Nematic Transition in Amyloid Fibril Suspensions Driven by Thermophoresis
 The isotropic and nematic (I + N) coexistence for rod-like colloids is a signature of the first-order thermodynamics nature of this phase transition. However, in the case of amyloid fibrils, the biphasic region is too small to be experimentally detected, due to their extremely high aspect ratio. Herein, we study the thermophoretic behaviour of fluorescently labelled β-lactoglobulin amyloid fibrils by inducing a temperature gradient across a microfluidic channel. We discover that fibrils accumulate towards the hot side of the channel at the temperature range studied, thus presenting a negative Soret coefficient. By exploiting this thermophoretic behaviour, we show that it becomes possible to induce a continuous I-N transition with the I and N phases at the extremities of the channel, starting from an initially single N phase, by generating an appropriate concentration gradient along the width of the microchannel. Accordingly, we introduce a new methodology to control liquid crystal phase transitions in anisotropic colloidal suspensions. Because the induced order-order transitions are achieved under stationary conditions, this may have important implications in both applied colloidal science, such as in separation and fractionation of colloids, as well as in fundamental soft condensed matter, by widening the accessibility of target regions in the phase diagrams.
The isotropic and nematic (I + N) coexistence for rod-like colloids is a signature of the first-order thermodynamics nature of this phase transition. However, in the case of amyloid fibrils, the biphasic region is too small to be experimentally detected, due to their extremely high aspect ratio. Herein, we study the thermophoretic behaviour of fluorescently labelled β-lactoglobulin amyloid fibrils by inducing a temperature gradient across a microfluidic channel. We discover that fibrils accumulate towards the hot side of the channel at the temperature range studied, thus presenting a negative Soret coefficient. By exploiting this thermophoretic behaviour, we show that it becomes possible to induce a continuous I-N transition with the I and N phases at the extremities of the channel, starting from an initially single N phase, by generating an appropriate concentration gradient along the width of the microchannel. Accordingly, we introduce a new methodology to control liquid crystal phase transitions in anisotropic colloidal suspensions. Because the induced order-order transitions are achieved under stationary conditions, this may have important implications in both applied colloidal science, such as in separation and fractionation of colloids, as well as in fundamental soft condensed matter, by widening the accessibility of target regions in the phase diagrams.
Publications:
D. Vigolo, J. Zhao, S. Handschin, X. Cao, A. J. deMello and R. Mezzenga. Continuous Isotropic-Nematic Transition in Amyloid Fibril Suspensions Driven by Thermophoresis. Scientific Reports, 2017, 7, 1211; doi:10.1038/s41598-017-01287-1